The U.S. Food and Drug Administration approved Journavx (suzetrigine) 50 milligram oral tablets, a first-in-class non-opioid analgesic, to treat moderate to severe acute pain in adults.
Journavx reduces pain by targeting a pain-signaling pathway involving sodium channels in the peripheral nervous system, before pain signals reach the brain.
Journavx is the first drug to be approved in this new class of pain management medicines.
Pain is a common medical problem and relief of pain is an important therapeutic goal. Acute pain is short-term pain that is typically in response to some form of tissue injury, such as trauma or surgery. Acute pain is often treated with analgesics that may or may not contain opioids.
The FDA has long supported development of non-opioid pain treatment. The agency aimed at encouraging development of non-opioid analgesics for acute pain and awarded cooperative grants to support the development and dissemination of clinical practice GUIDELINESfor the management of acute pain conditions.
“Today’s approval is an important public health milestone in acute pain management,” said Jacqueline Corrigan-Curay, J.D., M.D., acting director of the FDA's Center for Drug Evaluation and Research. “A new non-opioid analgesic therapeutic class for acute pain offers an opportunity to mitigate certain risks associated with using an opioid for pain and provides patients with another treatment option. This action and the agency’s designations to expedite the drug’s development and review underscore FDA’s commitment to approving safe and effective alternatives to opioids for pain management.”
The efficacy of Journavx was evaluated in two randomized, double-blind, placebo- and active-controlled trials of acute surgical pain, one following abdominoplasty and the other following bunionectomy. In addition to receiving the randomized treatment, all participants in the trials with inadequate pain control were permitted to use ibuprofen as needed for “rescue” pain medication. Both trials demonstrated a statistically significant superior reduction in pain with Journavx compared to placebo.
The safety profile of Journavx is primarily based on data from the pooled, double-blind, placebo- and active-controlled trials in 874 participants with moderate to severe acute pain following abdominoplasty and bunionectomy, with supportive safety data from one single-arm, open-label study in 256 participants with moderate to severe acute pain in a range of acute pain conditions.
The most common adverse reactions in study participants who received Journavx were itching, muscle spasms, increased blood level of creatine phosphokinase, and rash. Journavx is contraindicated for concomitant use with strong CYP3A inhibitors. Additionally, patients should avoid food or drink containing grapefruit when taking Journavx.
The application received Breakthrough Therapy, Fast Track and Priority Review designations by the FDA.
The FDA granted approval of Journavx to Vertex Pharmaceuticals Incorporated.

 Applications open for new United Way of Central Indiana initiative to build community solutions
Applications open for new United Way of Central Indiana initiative to build community solutions
 Utilities District of Western Indiana REMC announces increases over next three years
Utilities District of Western Indiana REMC announces increases over next three years
 Fountain County man arrested on sex crime charges, Parke County investigation remains
Fountain County man arrested on sex crime charges, Parke County investigation remains
 Friday is National Wear Red Day
Friday is National Wear Red Day
 Vermillion County DCS worker facing felonies
Vermillion County DCS worker facing felonies
 One week left to file for office in Indiana
One week left to file for office in Indiana
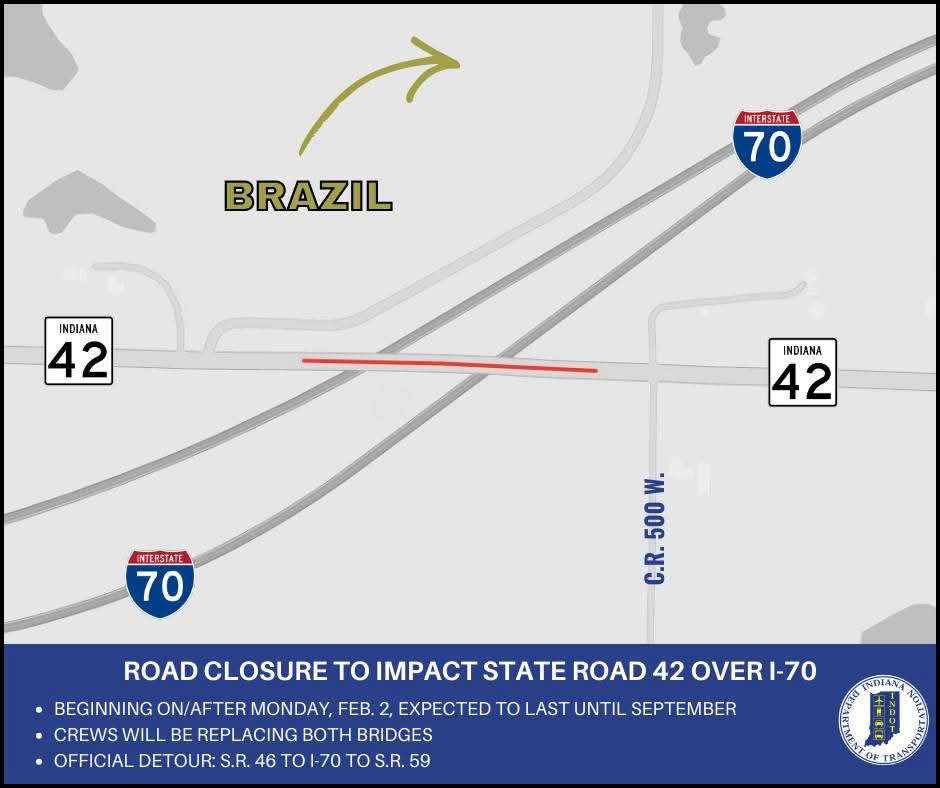 Bridge closures to impact State Road 42 over I-70 in Clay County
Bridge closures to impact State Road 42 over I-70 in Clay County
 Road renaming resolution co-authored by Criswell to honor Bobby Knight passes House
Road renaming resolution co-authored by Criswell to honor Bobby Knight passes House
 Thrive West Central to host Utility Workshop
Thrive West Central to host Utility Workshop
 Greene Realty continues to grow
Greene Realty continues to grow
 World Food Championships set for 2026 Indianapolis return
World Food Championships set for 2026 Indianapolis return
 SW Parke Community Schools looking at Revision Project
SW Parke Community Schools looking at Revision Project
 NWS extends Winter Storm Warning to Monday morning
NWS extends Winter Storm Warning to Monday morning
 IDHS activates State Emergency Operations Center in response to winter storm
IDHS activates State Emergency Operations Center in response to winter storm
 Scholarship Fund honors former Coach Blank
Scholarship Fund honors former Coach Blank
 Winter Storm Watch in effect for the weekend, heavy snow possible
Winter Storm Watch in effect for the weekend, heavy snow possible
 ISP releases Human Trafficking Awareness Initiative results
ISP releases Human Trafficking Awareness Initiative results